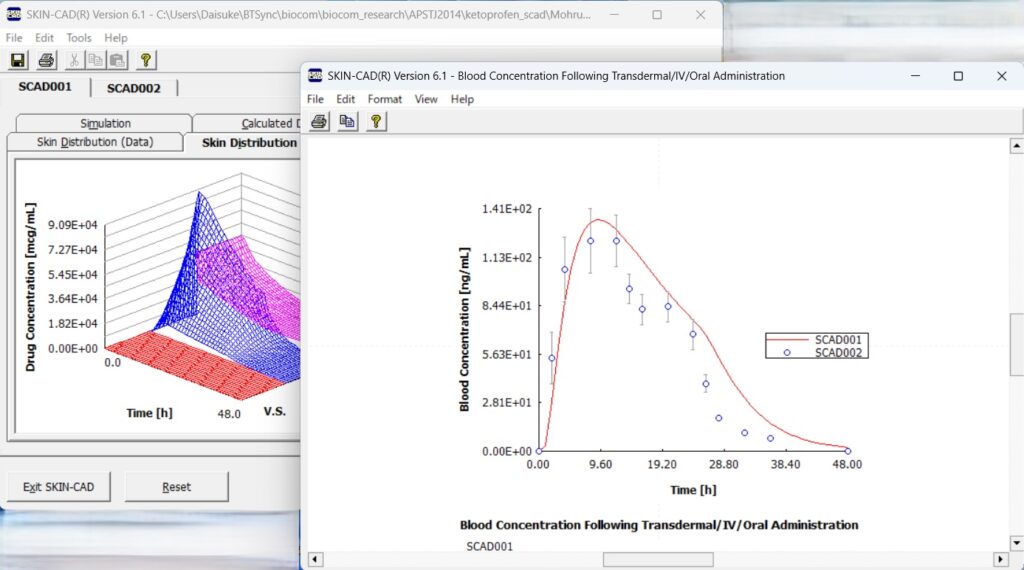
~ SKIN-CAD®はin vitro皮膚透過データから血中濃度推移を予測できます ~
SKIN-CAD®は経皮吸収モデルに基づいた皮内・血中薬物動態解析ソフトウェアです.
in vitro皮膚透過データや既知の全身PKパラメータなどを入力することで,臨床(in vivo)条件下での経皮吸収量や血中濃度の時間変化を算出します.
全身作用薬の血中濃度推移,局所治療薬の皮内薬物動態,化粧品有効成分の皮膚浸透量など,目的に応じて解析できます.
(監修:九州工業大学 東條角治 名誉教授)
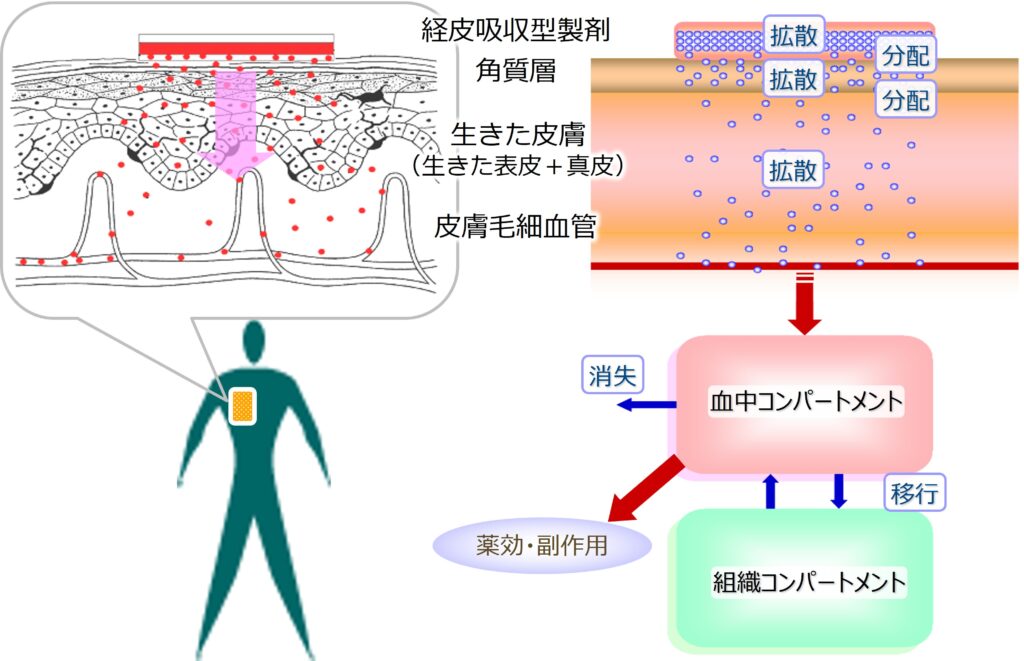
【経皮吸収モデル:皮膚拡散・分配/全身コンパートメントモデル】
主な入力パラメータ
<皮膚構造及び皮膚拡散・分配モデル>
・角質層厚み
・皮膚表面から毛細血管までの距離
・角質層内拡散係数
・生きた皮膚(生きた表皮・真皮)内拡散係数
・角質層/生きた皮膚分配係数
<全身コンパートメントモデル>
(1-/2-/3-コンパートメントモデル)
・分布容積
・消失速度定数
・コンパートメント間移行速度定数
【弊社が関わったSKIN-CAD®関連の論文,学会発表など】
- 森 (バイオコム), 宮城 (就実大), 引間 (九州工大): 局所皮膚適用貼付剤の生物学的同等性評価に及ぼす影響因子, 日本薬学会第145年会, 福岡, 2025年3月29日.
- 森 (バイオコム), 引間 (九州工大): 経皮吸収型製剤のin vitro/in silico評価法 (第Ⅱ編 医薬品業界 第6章), 動物実験代替法とNew Approach Methodologiesの開発・利用動向, シーエムシー出版, 2023.
- 森 (バイオコム) : 経皮吸収予測におけるin vitro-in silicoアプローチ, 谷本学校 毒性質問箱 第20号, 安全性評価研究会, 2018.
- Hikima, T.1; Komatsu, M.1; Mori, D.2; Tojo, K.1 (1 Kyushu Institute of Technology, 2 Biocom Systems) Prediction of Percutaneous Absorption in Human of Nicotine from Marketed TTS using Three-dimensional Human Cultured Epidermis, 40th Annual Meeting & Exposition of the Controlled Release Society, Honolulu, Hawaii, July 21-24, 2013.
- Al-Qallaf, B.1; Das, D.B.1; Mori, D.2; Cui, Z.1 (1 Oxford University, 2 Biocom Systems) Modelling transdermal delivery of high molecular weight drugs from microneedle systems, Phil. Trans. R. Soc. A 2007, 365(1861), 2951–2967.
その他多数
【SKIN-CAD®納入実績】
(国内)
久光製薬株式会社 様
日東電工株式会社 様
トーアエイヨー株式会社 様
大正製薬株式会社 様
国立がん研究センター 様
など
(海外)
大手製薬企業(欧州)
大手製薬企業(米国)
Loughborough Univ. (UK)
など
【弊社以外でSKIN-CAD®を使用した企業や大学の研究成果】
- Miwa, Y.1; Hamamoto, H.1; Ishida, T.2 (1MEDRx, 2Tokushima University) Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid, Eur. J. Pharm. Biopharm. 2016, 102, 92-100.
- Ishida, M.; Takeuchi, H.; Endo, H.; Yamaguchi, J.-I. (Taisho Pharmaceutical) Impact of Humidity on In Vitro Human Skin Permeation Experiments for Predicting In Vivo Permeability, J. Pharm. Sci., 2015, 104, 4223–4231.
- 岩男美宏1; 南 邦弘2 (1日東電工, 2トーアエイヨー) ゴム系粘着剤を用いた経皮吸収型・β1遮断剤の製剤設計, 日本ゴム協会誌 2014, 87(10), 422-427.
- 岩男美宏1; 松岡賢介1; 岡田勝博1; 南 邦弘2; 湯浅修一朗2 (1日東電工, 2トーアエイヨー) ビソプロロール経皮投与デバイス, 特開2013-199486, 2013.
- 武内博幸1; 石田真大1; 浦野英俊1; 杉林堅次2 (1大正製薬, 2城西大学) Yucatan micropig皮膚を介したin vitro透過性からニコチンテープまたはリドカインテープをヒトに適用後の血中濃度の予測, 薬剤学 2012, 72(4), 251-261.
- Olatunji, O.; Das, D.B.; Nassehi, V. (Loughborough University) Modelling transdermal drug delivery using microneedles: Effect of geometry on drug transport behaviour, J. Pharm. Sci. 2012, 101(1), 164-175.
- 立石哲郎; 天野智史; 本間佐知子; 肥後成人 (久光製薬) 貼付剤, 特許第4694967号, 2011.
- 佐伯有史; 松岡賢介; 村岡崇光; 保坂美文; 堀 光彦; 二宮和久; 明見 仁; 黒田英利 (日東電工) フェンタニル含有貼付製剤, 特許第4745747号, 2011.
- 寺原孝明; 利光新太; 上村健吾; 肥後成人; 後藤 武; 佐藤秀次 (久光製薬) 経皮吸収型製剤, 特許第4394443号, 2010.
- Yoshitake, M.; Ga, K. (Yutoku Pharmaceutical Industries) Riluzole-containing transdermal patch, WO2010113225 A1, 2010.
| 稼働環境 | ・対応OS:Windows 10 / 11 ・ハードディスク必要空き容量:約20MB ・CD-ROMドライブ(CDからインストールする場合に必要です) ・インターネットブラウザ(ヘルプ閲覧に必要です) |
| 価格 | 年間ライセンス (初年度導入費,次年度以降の年間保守費を設定しています) (詳しくは弊社または販売代理店にお問い合わせください) |
| 販売代理店 | 販売代理店(日本):インフォコム株式会社 医薬IT・サイエンス事業部 サイエンスグループ 販売代理店(海外):FQS Poland Sp. z o.o.(富士通株式会社 子会社) |
【受託計算サービス】
SKIN-CAD®による受託計算サービスを承ります.
「皮膚透過データはあるが血中濃度を予測する方法が分からない」「SKIN-CADを操作する人員がいない」「使い方を習得する時間が無い」「解析する事例数が少ない」という方は是非お問い合わせください.お持ちの in vitro皮膚透過データ等から血中濃度や皮内濃度を予測します.(必要に応じて秘密保持契約を締結致します)
